ethanol lewis dot|Ethanol Lewis Dot Structure: Drawing And Detailed Explanations : Bacolod Lewis structure of C2H5OH (or Ethanol) contains five C-H bonds, one O-H bond and one C-O bond. The two Carbon atoms (C) are at the center and it is surrounded by Hydrogen atoms (H) and one OH group. The oxygen . Kerolay Chaves Porn Videos white fairy elisa sanches adriana olivarez isabela ramirez aline novak andressa urach valeria castañeda brida nunes marcia imperator bruna ferraz. kerolay chaves all Trending New Popular Featured. HD 720p 1080p 4k All. Duration 10+min 20+min 40+min All. Date
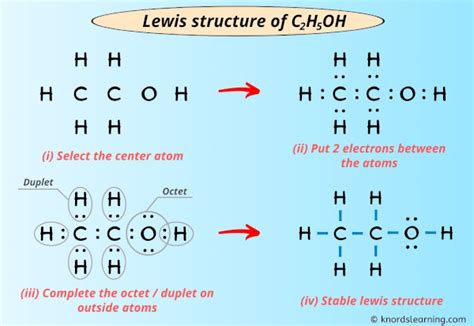
ethanol lewis dot,A step-by-step explanation of how to draw the C2H5OH Lewis Dot Structure (Ethanol (Ethyl alcohol)). For the C2H5OH structure use the periodic table to find the total number of valence. I quickly take you through how to draw the Lewis Structure of CH3CH2OH (Ethanol). I also go over hybridization, shape, sigma, pi bonding and bond angles.
What is the Lewis dot structure for ethanol (CH3CH2OH)? The Lewis dot structure for ethanol ( CH3CH2OH ) shows that the two carbon atoms are bonded together, with one .Ethanol (CH 3 CH 2 OH) Lewis Structure. Ethanol (CH 3 CH 2 OH) contains two carbon atoms, six hydrogen atoms and one oxygen atom. Two carbon atoms have joint with a single bond .
ethanol lewis dot Lewis structure of C2H5OH (or Ethanol) contains five C-H bonds, one O-H bond and one C-O bond. The two Carbon atoms (C) are at the center and it is surrounded by Hydrogen atoms (H) and one OH group. The oxygen .
Lewis Dot of Ethanol (Ethyl Alcohol) CH 3 CH 2 OH. Back. 70 More Lewis Dot Structures. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The . { "name":"jmolApplet0_object","applet":true,"documentBase":"https://www.chem.purdue.edu/jmol/molecules/ch3ch2oh.html","platform":"J.awtjs2d.Platform","fullName . As the valence electrons in an Oxygen atom are 6, when oxygen shares two electrons, one each with Carbon and Hydrogen, it is left with two lone pairs of electrons. This is the most stable and accepted Lewis dot structure of . An explanation of the molecular geometry for the C2H5OH (Ethanol) including a description of the C2H5OH bond angles.Lewis Symbols. At the beginning of the 20 th century, an American physical chemist G. N. Lewis (1875–1946) devised a system of symbols—now called Lewis electron dot symbols (often shortened to Lewis dot symbols) that can be used for predicting the number of bonds formed by most elements in their compounds.Each Lewis dot symbol consists of the chemical symbol .
A step-by-step explanation of how to draw the C2H6O Lewis Dot Structure. Three are two ways to draw the Lewis structure for C2H6O. Both use all 20 valence e.
CH 3 CH 2 OH or C 2 H 5 OH or C 2 H 6 O (ethanol) has two carbon atoms, six hydrogen atoms, and one oxygen atom.. In the ethanol Lewis structure, there are five C — H bonds, one C — C bond, one C — O bond, .How to draw Lewis structures. Given a chemical formula corresponding to a molecule or molecular ion, the steps to obtain its Lewis structure are as follows: First, it is important to get a correct count of all the valence electrons. One way to do such a count is to write Lewis symbols for all the atoms in the formula and count all the “dots.” A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.

{ "name":"jmolApplet0_object","applet":true,"documentBase":"https://www.chem.purdue.edu/jmol/molecules/ch3ch2oh.html","platform":"J.awtjs2d.Platform","fullName .
Infact, I’ve also given the step-by-step images for drawing the lewis dot structure of C2H5OH molecule. So, if you are ready to go with these 6 simple steps, then let’s dive right into it! Lewis structure of C2H5OH (or Ethanol) contains five .
Formula Lewis Electron-Dot Diagram : Ethanethiol CH 3 CH 2 SH : Ethane : CH 3 CH 3: Ethanol CH 3 CH 2 OH : Ethyne : C 2 H 2 (a) Draw the complete Lewis electron-dot diagram for ethyne in the appropriate cell in the table above. See the lower right cell in the table above. One point is earned for the correct Lewis structure.Transcript: So there are two ways presented here to draw the C2H6O Lewis structure. On the left, we have the Oxygen atom between the two Carbons. This is called dimethyl ether. On the right, the Oxygen atom's on the outside with the Hydrogen attached to it. That's called ethanol. So they're both valid Lewis structures. The drawing of Lewis electron-dot structures is guided largely by the octet rule: that atoms form bonds to achieve eight electrons in their valence shell. For many elements, a full valence shell has an electron configuration of \(s^2p^6\), or eight electrons. A common exception to this rule is the first row elements, H and He. These two .
ethanol lewis dot Ethanol Lewis Dot Structure: Drawing And Detailed Explanations Example \(\PageIndex{1}\): Lewis Structures. Solution; Lewis used simple diagrams (now called Lewis diagrams) to keep track of how many electrons were present in the outermost, or valence, shell of a given atom.The kernel of the atom, i.e., the nucleus together with the inner electrons, is represented by the chemical symbol, and only the valence electrons are . CH3OH Lewis Structure. The Lewis Structure of a molecule gives the simplest representation of valence shell electrons around itself. Here, the valence electrons are represented by small dots and since a single bond .Ethanol Lewis Dot Structure: Drawing And Detailed Explanations Lewis structures show all of the valence electrons in an atom or molecule. . To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that . Lewis dot symbols provide a simple rationalization of why elements form compounds with the observed stoichiometries. In the Lewis model, the number of bonds formed by an element in a neutral compound is the same as the number of unpaired electrons it must share with other atoms to complete its octet of electrons.
The Lewis dot structure of ethanol (C 2 H 5 OH) displays a total of 20 valence electrons i.e., 20/2 = 10 electron pairs. Two carbon (C) atoms, single-bonded to each other at the center, are simultaneously bonded to three H-atoms on one side and two H-atoms, and an OH functional group on the other side.The lewis dot structure of ethanol is given below. Estimate the enthalpy of combustion of ethanol (C2H5OH) using the bond enthalpies in the table. Show transcribed image text. There are 2 steps to solve this one.Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.

The Lewis structure of ethanol CH 3 CH 2 OH contains eight single bonds, with two carbons in the center, and oxygen and six hydrogens on either side. There are two lone pairs on the oxygen atom, and carbon atom and hydrogen atom do not have any lone pair.
ethanol lewis dot|Ethanol Lewis Dot Structure: Drawing And Detailed Explanations
PH0 · Lewis Structure of C2H5OH (Ethanol) (With 6 Simple
PH1 · Lewis Dot of Ethanol CH3CH2OH
PH2 · Lewis Dot Structure of CH3CH2OH (Ethanol)
PH3 · How to Draw the Lewis Dot Structure for C2H5OH: Ethanol
PH4 · Ethyl Alcohol Formula & Structure
PH5 · Ethanol Lewis Dot Structure: Drawing And Detailed Explanations
PH6 · Ethanol (CH3CH2OH) Lewis Structure
PH7 · C2H5OH Lewis Structure, Molecular Geometry, Bond Angles and Hybrid
PH8 · C2H5OH Lewis Structure, Molecular Geometry, Bond Angles and
PH9 · C2H5OH Lewis Structure, Molecular Geometry, Bond
PH10 · C2H5OH Lewis Structure, Molecular Geometry
PH11 · C2H5OH (Ethanol): Molecular Geometry and Bond Angles